Nuclear fission is a phenomenon in which a heavy nucleus, splits into two smaller nuclei, called the fission products, mostly of unequal masses, one often with nearly half the mass as the other, and rarely of equal masses. This reaction gives off a large amount of energy and emits two or more neutrons, and gamma rays. Fission can occur spontaneously, and it could be induced by bombarding the heavy nucleus with particles like neutron. The probability of spontaneous fission is very low. In the context of atomic energy (i.e. nuclear energy), fission implies neutron-induced fission. When a neutron hits a heavy nuclide like U-235, the neutron gets absorbed in the heavy nuclide that gets energetically agitated (or excited). If the new energy state of the heavy nuclide is sufficient for it to split, then it can split to cause fission. The probability of fissioning depends on the type of heavy nuclide, and is sensitive to neutron energy. Further, when neutron hits a heavy nuclide, fission is one of the various reactions, each with an associated probability of occurrence. In other words, every collision of a neutron and a heavy nuclide does not necessarily result in fission. The nuclear energy released in fission is about a million times the chemical energy released in burning a block of coal of equal mass. About 80 % of the energy released in fission are carried away by the fission products (and the rest by the other particles), which in turn transfer the energy to the surroundings, making the energy recoverable. As a fission caused by a neutron involves production of further neutrons, a fission chain reaction becomes possible, and such a chain is ensured in the design of a reactor.
The term nuclear fusion refers to the reaction in which two very light nuclei are bound to form a heavier, more stable nucleus with a large release of energy.
In order for fusion to take place, the positively charged nuclei should approach each other by overcoming the electrostatic forces of repulsion. The kinetic energy required for the nuclei that react to overcome these interactions can be supplied in the form of thermal energy or by using a particle accelerator
3.What are the fuels used to produce nuclear energy?
Naturally occurring uranium contains 0.71% of the isotope U -235, the rest mainly being the isotope U -238. Of all the naturally occurring materials only the rare isotope U -235 can sustain а fission chain reaction and is called fissile material. Other fissile materials, which can fuel nuclear reactors, are plutonium and U -233. These latter materials are man-made and do not occur in nature. Plutonium is created when U -238 is irradiated in а nuclear reactor where it absorbs neutrons which transmutes а part of the U -238 into plutonium. Similarly, U -233 is created when thorium is irradiated in а nuclear reactor, where it absorbs neutrons, which transmutes а part of the thorium into U-233. Thus U-238 and thorium are also valuable nuclear resources, called fertile materials, as they can be converted into fissile material for fuelling nuclear reactors and producing energy.
4.What is the principle of Nuclear Reactor?
The
nuclear reactor operates on the principle of nuclear fission, a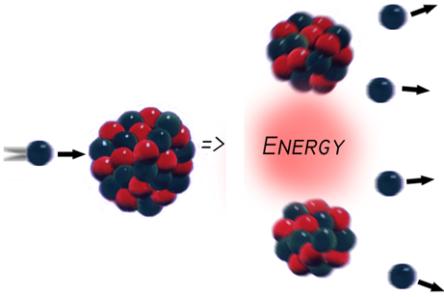 n event that
occurs when an atom is struck by a neutron and flies apart.
n event that
occurs when an atom is struck by a neutron and flies apart.
The figure depicts a neutron heading towards a fissile atom (one that is capable of supporting fission). Most often, this is Uranium-235 or Plutonium-239.
When the high-velocity neutron strikes the nucleus, the atom sheds more neutrons and breaks up into several other particles. The motion of all these particles and atoms flying around and colliding creates the heat that is used in power plants to generate electricity.
![]()
5. What is the advantage of Nuclear Technology?
Radioactive materials benefit us in many ways. Besides producing electricity, they are used to diagnose and treat diseases, including cancer. They also are used to test new drugs for safety, to kill bacteria in food, to help explore for oil and gas, to breed farm crops that are more resistant to disease, to provide electricity for unmanned spacecraft and to power ships and submarines. (Please roll over the various objects below to obtain more information.)
courtesy : www.nucleartourist.com
7. Where are the nuclear power stations producing electric power located in India? What is the total electric power produced from nuclear energy in India and the breakup for different stations? Are more nuclear power reactors going to be installed in India in the next five years?
The atomic power stations which are already commissioned in India are located and produce power as indicated below:
а) Tarapur Atomic Power Station, Tarapur, Maharasthra, 2 BWRs, now rated at 160 MWe each.
b) Rajasthan Atomic Power Station, Kota, Rajasthan, 4 PHWRs, the first two now rated at 100 MWe and 200 МWе, and the later two at 220 MWe each.
с) Madras Atomic Power Station, Каlpakkam, Tamilnadu, 2 PHWRs, now rated at 170 MWe each.
d) Narora Atomic Power Station, Narora, UP, 2 PHWRs of 220 MWe each.
е) Kakrapar Atomic Power Station, Kakrapar, Gujarat, 2 PHWRs of 220 МWе each.
f) Kaiga Generating Station, Kaiga, Karnataka, 2 PHWRs of220 МWе each.
The total installed nuclear electric gross capacity works out to be 2720 MWe while the net capacity (allowing for station consumption) is 2503 MWe.
Additional PHWRs are under construction as follows:- 2х500 Mwe at Tarapur, 2х220 MWe Коtа and 2х220 MWe at Kaiga. Two 1000 Me PWRs of the Russian VVER type are also being set up with Russian collaboration at Kudankulam (Tamilnadu). А 500 MWe Prototype Fast Breeder Reactor is under construction at Kalpakkam.
It is expected to reach а nuclear capacity of 20,000 MWe by year 2020.
8. In which states of India are uranium and thorium located?
Uranium is found in some parts of India, the major portion of the known reserves being located in Bihar. At present, the uranium mineral deposits at Jaduguda, Bihar are being commercially exploited by the Uranium Corporation of India Ltd. (UCIL). Other than Bihar, target areas have been identified in Meghalaya, Karnataka and Andhra Pradesh. In addition, uranium has also been located in copper deposits and is recovered as а by-product in copper-ore processing.
Thorium is contained in the mineral monazite, which occurs in beach sand deposits on the coasts of Kerala, Tamilnadu and Orissa. It is processed by Indian Rare Earths Ltd. (IRE).
9. What are the types of Nuclear reactors in India?
Pressurized Heavy Water Reactor (PHWR) – MAPS, RAPS, KAPS, NAPS, KAIGA
Boiling Water Reactor (BWR) – TAPS
Fast Breeder Reactor (FBR) – PFBR
V V E R -1000 (PWR) – Kundankulam
10. How is plutonium produced and used and how is nuclear waste disposed of ?
Spent fuel discharged from reactors is not considered а waste as it contains unused U-238 as well as plutonium. The spent fuel is therefore sent to reprocessing plants for recovery of plutonium and uranium. At these plants the spent fuel is dissolved in acid and all the radioactive materials go into solution. Plutonium and uranium are separated from this solution which retains the radioactive fission products. The separated plutonium and uranium are then processed appropriately for re-use in reactors. The solution containing radioactive fission products is treated as high level liquid waste and is stored in stainless steel tanks in underground vaults. The vaults are lined. inside with stainless steel plate and are isolated from ground water outside. After storage here for an interim period, the liquid is processed and finally converted to solid glass, encased in metal cans and stored in an underground repository. These techniques have been already developed indigenously and tested.
The concentration of radioactive materials in high level solid wastes from reprocessing plants fixed in glass blocks is expected to decrease by а factor of 1000 in about 300 years. А further 1000-fold decrease is expected over the next 4000 years. At this stage, the radioactive material content in the glass block is likely to be within about 100 times that in uranium ore. This radioactivity would be primarily due to residual plutonium which has а half life of 24,000 years. The long life of the high level solid waste is not of concern as it is stored in the form of glass in containers with multiple walls, at locations carefully selected to rule out contact with ground water. The leaching of plutonium from the glass is so small as to be negligible even over tens of thousands of years. Further, the volume of high level solid waste is very small; one full day's operation at the MAPS produces only one small bucketful of this waste.