41. Up to what distance from the atomic reactor, is radioactivity monitored? What are the methods used and safeguards provided?
Environmental Survey Laboratories are set up at nuclear power plant sites well before the reactors start operating. These laboratories investigate and establish the base line radioactivity levels in and around the site before the plant commences operation and then continue to monitor the area to ensure that there is no undue increase in radioactivity subsequently. The food and water pathways leading to radioactivity exposure by the population around the site are determined and monitoring methods are established to check that there is no increase in this exposure due to the power plant operations. An Environmental Survey Laboratory has been in operation at Kalpakkam since 1974 while the first MAPS reactor commenced operations only since 1983. The laboratory has investigated radioactivity levels in materials relevant to radiation exposure of humans (e.g. cereals, vegetables, milk, sea-food, water, air, salt etc). Monitoring of the radioactivity levels in these materials is done on а continuing basis up to 32 km radial distance from the power plant. Ambient radiation levels are checked with radiation meters and quarterly exposure doses are measured using thermo-luminescent dosimeters at а number of stations spread over the 32 km radial zone. Samples collected from the field are processed in the laboratory and investigated for radioactivity levels using radiochemical analytical methods or advanced nuclear counting systems. These levels are directly compared with the base line levels established before MAPS started operating. With the help of the dietary intake statistics compiled for the Ка1раккат area, the annual intake of radioactivity for а representative member of the population and consequent exposure dose are computed using the measured radioactivity levels in materials of the food chain and in the environment. These are compared with evaluated exposure dose of the pre-operational phase. These methods have provided valuable data and demonstrated that MAPS operation has had а very low and negligible radiological impact on the environment. Similar results have been established at other nuclear power station sites in India.
42. In day-to-day life, in what areas and articles of daily use does one come across radioactivity? What are the other areas where nuclear energy is used?
Radiation has always been present everywhere in the environment. Every human being is also naturally radioactive as potassium is an essential constituent of body tissues and fluids, and contains а small amount of the radioactive isotope К-40. In а small area of land 40 m х 10 m surrounding а typical house there is contained in the soil down to 1 m depth, 2 kg of uranium, б kg of thorium and 0.8 kg of К-40, all of which are radioactive along with associated radioactive daughter products. Of course these concentrations of U and Th are too low for economic recovery! Not only are radioactive materials like uranium, thorium, radium and potassium present in earth everywhere, radiation is constantly streaming towards earth from outer space in the form of cosmic radiation. Our bodies also contain radioactive С-14, which is produced in the atmosphere by the action of cosmic rays and absorbed by living organisms. In aero plane flights passengers are exposed to higher than normal radiation levels on account of the increased cosmic radiation (at 1640 m elevation the cosmic ray dose is approximately double that at sea level) .
The natural radiation background from the natural radioactivity in the earth and from cosmic rays varies from place to place by as much as а factor ten. The radiation exposure to the population around nuclear power plants and attributable to its operation is found to be about а factor 30 times lower than the average natural background radiation exposure.
Besides the production of electric power, nuclear energy is used for heat supply to process industries as well as in power packs for transportation (ships, submarines and spacecraft) . There are in addition many other uses of radioactivity and nuclear radiation . Important applications in medicine, agriculture, research and industry make use of the following properties of nuclear radiation :
- they can promote chemical and physico-chemical
changes,
- they can destroy bacteria, change living cell activity and cause genetic
mutations,
- they are absorbed, transmitted or reflected differently by different
materials,
- they can be detected in extreme dispersion,
- they can generate heat and electric current.
Radiation therapy is а well established procedure of modern medicine, while radioactive tracers are used both in medicine and industry. Neutron and gamma radiography are now commonly used methods of nondestructive characterization and evaluation in industry and research. Standard irradiation procedures exist for increasing the shelf life of agricultural products, sterilization of equipment, polymerization of molecules etc. Use of radiation to cause genetic changes along with selective breeding has led to the development of high yielding crop varieties, which have been adopted in this country.
Radio isotopes needed for all the above uses are produced in the research reactors located at the Bhabha Atomic Research Centre (ВАRС), Mumbai and the Variable Energy Cyclotron Centre (VECC), Kolkata. In addition R & D on all these multifaceted uses of radioactivity and nuclear radiation is carried out at these centres .
43. What are the developments foreseen in the use of nuclear power in India in the coming years?
The development activities in the area of power production are mainly on three fronts. First is the thrust towards the establishment of an expanded PHWR power production capacity of the order of 10,000 MWe over the next decade. This involves development of а 700 MWe PHWR design as well as standardization of PHWR reactor components to achieve economy of scale and mass production. The R & D towards this task are carried out in the Nuclear Power Corporation (NPCIL) and BARC.
On the second front is the challenging task of establishing fast breeder technology in this country so as to be able to efficiently convert U-238 and thorium into fissile material and be technically capable of setting up nuclear electric capacities of а few hundred thousand MWe in tune with the country's requirements in the 21st century. The R & D towards this goal is carried out at the Indira Gandhi Centre for Atomic Research (IGCAR), Kalpakkam
On the third front is the R & D towards advanced technologies such as lasers, accelerators and fusion so as to be able to exploit the developments in the energy conversion technologies of the future. The work in this direction is carried out at ВАRС and at the Centre for Advanced Technology (CAT), Indore.
Two types of process are there for enrichment of Uranium.
1. Gaseous Diffusion 2. Gas centrifuge
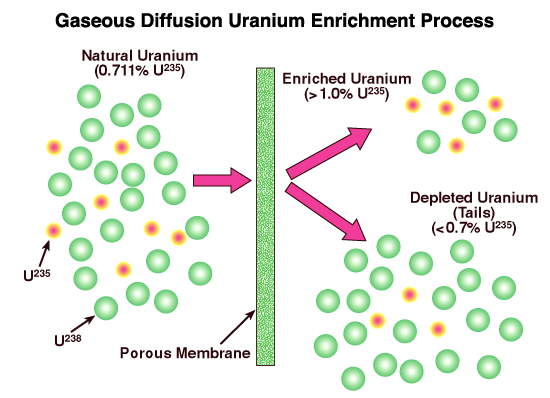
In the gaseous diffusion enrichment plant, the solid uranium hexafluoride (UF6) from the conversion process is heated in its container until it becomes a liquid. The container becomes slightly pressurized as the solid melts. Because the container is not completely full UF6 gas then fills the top of the container. The UF6 gas is slowly fed into the plant’s pipelines where it is pumped through special filters called barriers or porous membranes. The holes in the barriers are so small that there is barely enough room for the UF6 gas molecules to pass through. The isotope enrichment occurs when the lighter UF6 gas molecules (with the uranium-234 (U234) and U235 atoms) tend to diffuse faster through the barriers than the heavier UF6 gas molecules containing uranium-238 (U-238). One barrier isn’t enough to do the job, though. It takes many hundreds of barriers, one after the other, before the UF6 gas contains enough uranium-235 to be used in reactors. At the end of the process, the enriched UF6 gas is withdrawn from the pipelines and condensed back into a liquid that is poured into containers. The UF6 is then allowed to cool and solidify before it is transported to fuel fabrication facilities where it is turned into fuel assemblies for nuclear power reactors.
The gas centrifuge uranium enrichment process uses a large number of rotating cylinders in a series. These series of centrifuge machines, called trains, are interconnected to form cascades. In this process, uranium hexafluoride (UF6) gas is placed in a rotating drum or cylinder and rotated at a high speed. This rotation creates a strong gravitational field so that the heavier gas molecules (containing U-238) move toward the outside of the cylinder and the lighter gas molecules (containing U-235) collect closer to the center. The stream that is slightly enriched in U-235 is withdrawn and fed into the next higher stage, while the slightly depleted stream is recycled back into the next lower stage. Significantly more U-235 enrichment can be obtained from a single unit gas centrifuge than from a single unit gaseous diffusion barrier.
In principle, a fuel cell operates like a battery. Unlike a battery, a fuel cell does not run down or require recharging. It will produce energy in the form of electricity and heat as long as fuel is supplied.
A fuel cell consists of two electrodes sandwiched around an electrolyte. Oxygen passes over one electrode and hydrogen over the other, generating electricity, water and heat.
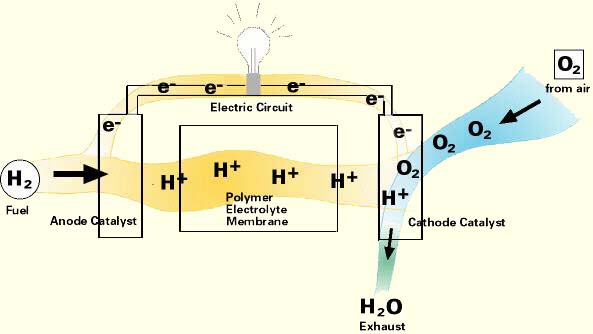
Hydrogen fuel is fed into the "anode" of the fuel cell. Oxygen (or air) enters the fuel cell through the cathode. Encouraged by a catalyst, the hydrogen atom splits into a proton and an electron, which take different paths to the cathode. The proton passes through the electrolyte. The electrons create a separate current that can be utilized before they return to the cathode, to be reunited with the hydrogen and oxygen in a molecule of water.
A fuel cell system which includes a "fuel reformer" can utilize the hydrogen from any hydrocarbon fuel - from natural gas to methanol, and even gasoline. Since the fuel cell relies on chemistry and not combustion, emissions from this type of a system would still be much smaller than emissions from the cleanest fuel combustion processes.
46. What is Prestressed Concrete?
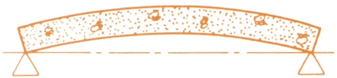
Prestressed Concrete is an architectural and structural material possessing great strength. The unique characteristics of prestressed concrete allow predetermined, engineering stresses to be placed in members to counteract stresses that occur when the unit is subjected to service loads. This is accomplished by combining the the best properties of two quality materials: high strength concrete for compression and high tensile strength steel strands for tension.
Actually, prestressing is quite simple. High tensile strands are stretched between abutments at each end of long casting beds. Concrete is then poured into the forms encasing the strands. As the concrete sets, it bonds to the tensioned steel. When the concrete reaches a specific strength, the strands are released from the abutments. This compresses the concrete, arches the member, and creates a built in resistance to service loads.
|
Prestressed Concrete
Beam |
 |
| The upward force along the length of the beam counteracts the service loads applied to the member. | 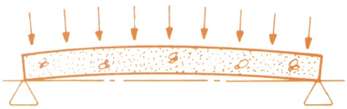 |
| Ordinary
Concrete Beam Even without a load, the ordinary concrete beam must carry its own considerable weight - this leaves only a portion of its strength available to resist added loads. |
 |
| Under service loads, the bottom of the beam will develop hairline cracks. | 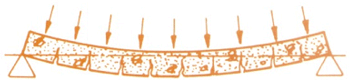 |
47. Which nuclei undergo fission?
Generally, all actinides (heavy nuclei starting
from Th-232) can undergo fission induced by neutron. Of these, nuclei beyond
uranium are unavailable in nature, but may be produced (artificially) in nuclear
transmutations. U-233, U-235, Pu-239, and Pu-241 are called fissile nuclides,
and can be fissioned with neutrons of any energy. Nuclides like Th-232, U-238,
etc., can undergo fission with neutron of certain minimum kinetic energy,
called threshold energy, below which the probability of fission is too small to
be significant. Other actinides undergo fission with varied probabilities. Many
of the actinides exhibit spontaneous fission. The spontaneous fission
probability of Cf-254 is significant.
![]()
48. What is spontaneous fission?
In the case of spontaneous fission, which is not caused by neutron, the original actinide of mass A itself fissions. Such a fission event must be understood as a mode of radioactive decay, like a decay. In other words, in a radioactive decay, just energy in the form of g rays, electrons (b particles), helium (a partilcles), etc., or, heavy nuclides like Xenon, Krypton, Barium, etc. could be emitted. U-238 mostly decays through a emission, while Cf-254 decays mostly through fission.
![]()
49. Which reactions are likely when a neutron hits a nucleus?
Many types of reactions are possible when a neutron hits a nucleus. The probability of each type of reaction happening depends on the nucleus and is very sensitive to the energy of the neutron. In this regard, two neighbouring nuclides (say, of masses A and A+1) may differ drastically. Some of the reactions are explained below:
Elastic scattering: The neutron and the nuclide collide and share a part of their kinetic energies. They rebound with speeds different from the original speeds, such that the ‘total kinetic energy’ before and after the collision remains the same. If the nucleus is stationary before collision, it will gain energy from the neutron and start moving, and the neutron gets slowed down due to loss of kinetic energy. This is the type of reaction that mostly helps fast neutrons to be slowed down to low energies in a reactor. Can the neutron gain energy? Yes. However, this is obviously possible when the nucleus has higher kinetic energy than the neutron. In a reactor, the average energy of a neutron produced in fission is about 2 MeV, whereas the average energy of the nuclei of the reactor materials is much below 1 eV. Therefore, the neutrons mostly undergo slowing down rather than gain energy. When the neutron is slowed to energies below the energies of the materials, the neutrons experience gaining energy as well. This would mostly happen below 1 eV, in a thermal reactor. In this range of energies, the neutrons and the nuclei would establish a thermal equilibrium.
Inelastic scattering: The neutron and the nuclide collide and rebound with speeds different from the original speeds, but the rebounding nuclide is left in an excited energy state. Hence the ‘total kinetic energy’ after the collision is less than that before the collision, and this difference accounts for the energy of excitation. If the nucleus is stationary before collision, the neutron must have kinetic energy exceeding the excitation energy, so that such a reaction is possible. Hence inelastic scattering is said to be a ‘threshold reaction’, the threshold being the minimum kinetic energy of the neutron required for the reaction to be possible. The excited nucleus subsequently de-excites by emitting g radiation. Heavy nuclides have lower thresholds than light nuclides. Though the probability of inelastic scattering is generally lower than elastic, the energy loss to the neutron is higher in an inelastic collision. Inelastic scattering in heavy nuclides degrades fission neutron energies heavily.
Capture: The neutron is absorbed by the target nucleus to form the next higher isotope (of mass A+1), in an excited state of energy. The new isotope de-excites by emitting g rays. The neutron is thus lost in this reaction. This is often known as ‘radiative capture’.
Fission: As explained earlier.
(n,x) reaction: In this reaction, ‘n’ represents neutron, and ‘x’ represents any particle like neutron, proton, deutron, a particle, etc. or a combination of such particles. It means that a neutron interaction with a nuclide results in emission of the particle(s) represented by ‘x’. (e.g.) if the emitted particle is a, it is called (n,a) reaction. If a neutron and a proton are emitted, then it is called (n,np) reaction. If 2 neutrons are emitted, it is then (n,2n) reaction. Such reactions are generally threshold reactions.
![]()
The term ‘cross-section’ generally refers to a plane surface or area of a cut-out section. The basic meaning of cross-section remains the same in the nuclear jargons as well. However, it is used to represent the probability of interaction between a projectile (a particle, say neutron that impinges on a target) and a target (say, an atom, that is hit by the projectile). Imagine a projectile approaching a target as in the figure below. Obviously the chance (probability) of a collision depends on the surface area projected by the target. i.e. The probability of collision is larger if the area is larger. If there are a large number of projectiles, the number of projectiles that could hit the surface is larger if the surface area is larger. Arguing in this manner, it is easy to visualize that the probability of collision (i.e. interaction) between the target and the projectile could be expressed by the effective surface area available for the collision.
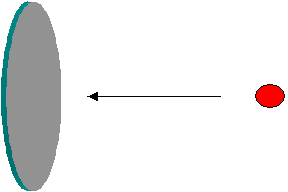
The idea is extended to represent probabilities of nuclear interactions, but a precise definition consistent with physics is employed. As the boundaries of sub-atomic systems are not sharp, the surface area supposed to be presented by a target can be larger than its geometric surface area. Thus it is now clear, in the nuclear jargons, that the term cross-section represents a measure of the ‘probability of interaction between a projectile and a target’. This quantity is usually called ‘microscopic cross-section’, represented by the symbol ‘s’, and is expressed in area units. The practical unit of microscopic cross-section is a ‘barn’, where 1 barn = 10-24 cm2 (i.e 10-28 m2).
A nuclear collision may result in a variety of reactions. As explained earlier, a neutron-nuclear collision could give rise to elastic or inelastic scattering, fission, or capture. The microscopic cross-section is accordingly defined to represent the measure of the probabilities of each such reaction. The reaction is indicated by a subscript in the symbol. Thus sel refers to elastic scattering, sin to inelastic scattering, sf to fission, sg (or s(n,g)) to radiative capture probabilities and so on. The symbol st called the ‘total cross-section’ is the sum of all the partial cross-sections.
The neutron-nuclear microscopic cross-sections vary significantly from nuclide to nuclide and drastically with respect to neutron energy. Knowledge of cross-sections is essential to understand the physics behind the design of a nuclear reactor and also to select the composition for reactor materials. Properly averaged cross-sections are usually used. The table below gives representative values of microscopic cross-sections for various materials of importance to thermal and fast reactors.
Table: Cross-sections of some important reactor materials
|
Material |
Cross-sections in barns |
||||
|
Thermal neutrons (E = 0.0253 eV) |
Fast neutrons (E > 100 eV) |
||||
|
Fission |
Capture |
Fission |
Capture |
||
|
Fissile |
92-U-233 |
5.28450E+02 |
4.57600E+01 |
2.73230E+00 |
2.71760E-01 |
|
92-U-235 |
5.85086E+02 |
9.86864E+01 |
1.90410E+00 |
5.55490E-01 |
|
|
94-Pu-239 |
7.47401E+02 |
2.70329E+02 |
1.79730E+00 |
4.96140E-01 |
|
|
Fertile |
92-U-238 |
1.17730E-05 |
2.71692E+00 |
4.27580E-02 |
3.31880E-01 |
|
90-Th-232 |
0.00000E+00 |
7.40000E+00 |
1.01930E-02 |
3.81570E-01 |
|
|
Clad |
40-Zr |
|
1.85396E-01 |
|
2.65766E-02 |
|
Steel |
|
3.08668E+00 |
|
1.70228E-02 |
|
|
Coolant |
Light Water |
|
6.64000E-01 |
|
5.15539E-04 |
|
Heavy Water |
|
1.30000E-03 |
|
1.14594E-04 |
|
|
11-Na-23 |
|
5.28000E-01 |
|
2.75110E-03 |
|
|
Control Rod |
5-B-10 |
|
3.84000E+03 |
|
2.73462E+00 |
|
48-Cd |
|
2.52415E+03 |
|
2.66766E-01 |
|
|
Fission Products |
54-Xe-135 |
|
2.63630E+06 |
|
5.99850E-03 |
|
36-Kr-83 |
|
2.07667E+02 |
|
2.35944E-01 |
|
|
62-Sm-149 |
|
4.01443E+04 |
|
1.91883E+00 |
|